Ensuring reliable and consistent monitoring of temperature and humidity is crucial for the proper storage of temperature-sensitive medications and perishable food products. Our partner, Vypin, has successfully deployed WhereView, a solution powered by ThingsBoard, for a leading global pharmaceutical corporation. This IoT application has significantly enhanced operational efficiency and cost-effectiveness.

The challenge
The Food and Drug Administration (FDA) imposes stringent guidelines on pharmaceutical manufacturers concerning storage conditions and the regulatory compliance of control systems. The client approached Vypin with a request to overhaul their existing system, which failed to meet FDA standards, leading to penalties and the potential for product or ingredient spoilage.
The client’s primary concerns revolved around data loss. The then-existing devices were prone to disconnections due to various issues, such as network or power failures, leading to lapses in telemetry transmission or recording. Furthermore, the system lacked a mechanism for notifying the client about these disruptions. Additionally, there was no provision for remote access to monitoring data.
Round-the-clock rotation of operators to control the temperature and humidity in the warehouse indeed is not the best solution. Therefore, in the regular automatic reports related to the storage environment state there were gaps in the tables and graphs. The FDA fines for such violations.
Customer officer says
The solution
In response, Vypin proposed the Vypin-WhereView system, leveraging ThingsBoard’s capabilities. The solution involved establishing a wireless network, along with the installation of sensors and gateways.
Addressing the initial concern of data loss, the newly installed sensors were equipped with memory modules to store telemetry data locally. Thus, even in the event of a gateway disconnection, data integrity was maintained. Upon restoration of the gateway’s online status, it would retrieve and transmit the stored telemetry to WhereView.
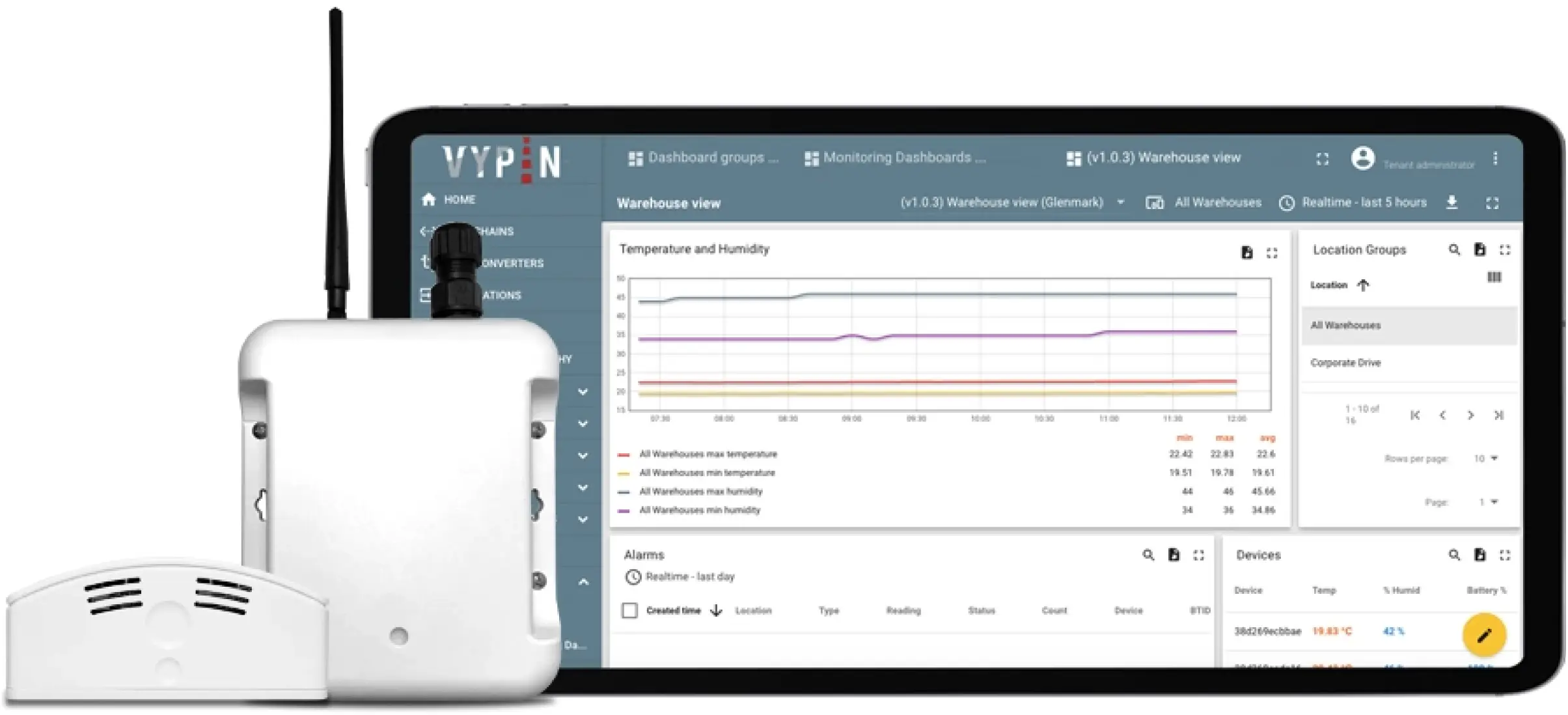
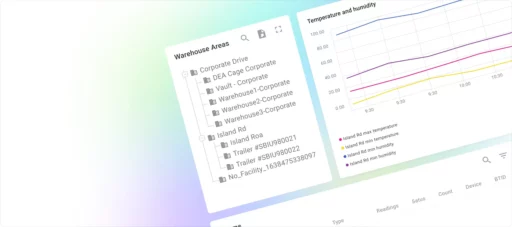
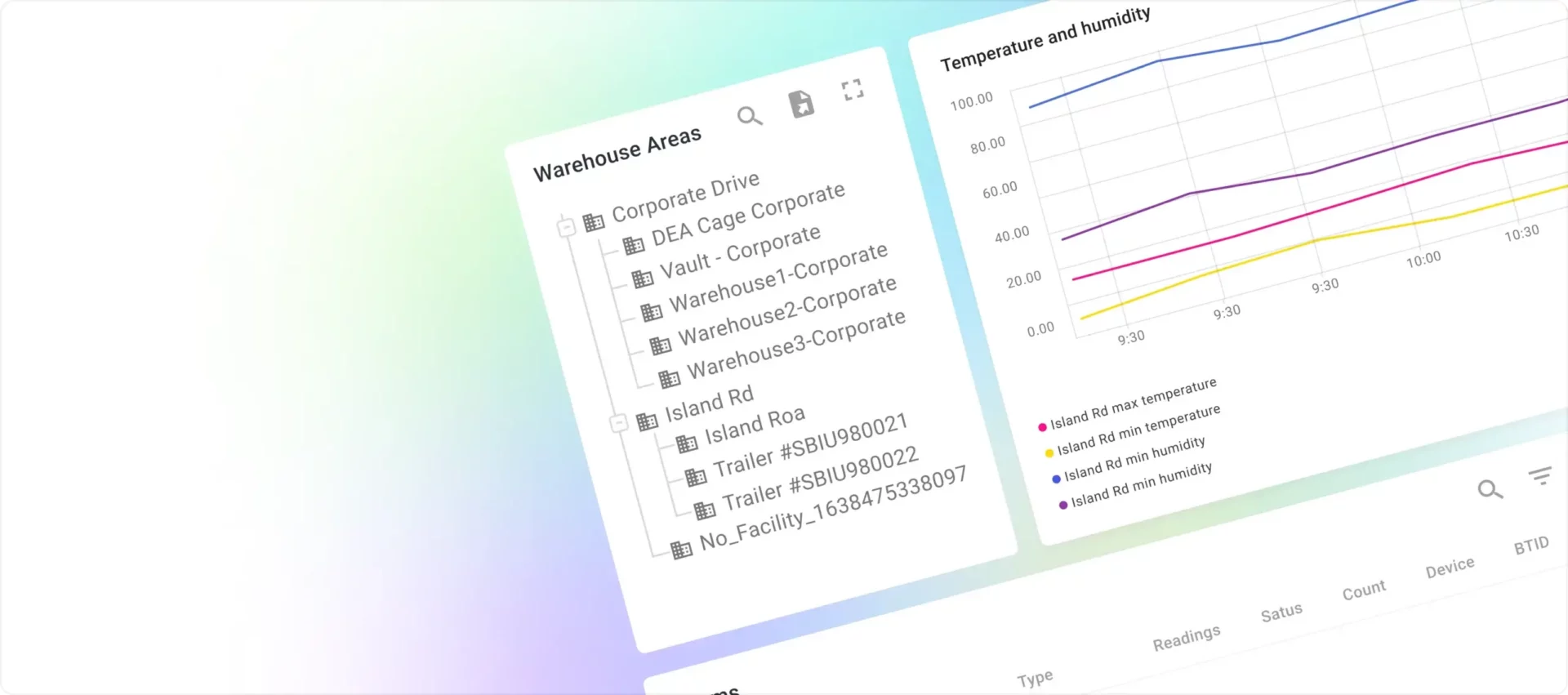
The WareView application, powered by ThingsBoard, resolved the outstanding concerns of reliable data processing and storage and enabled differentiated access levels. Designated personnel were granted comprehensive access, allowing them to monitor data from all warehouses at any time and location. In contrast, warehouse managers were provided with tailored access rights, enabling them to oversee only their specific warehouses.
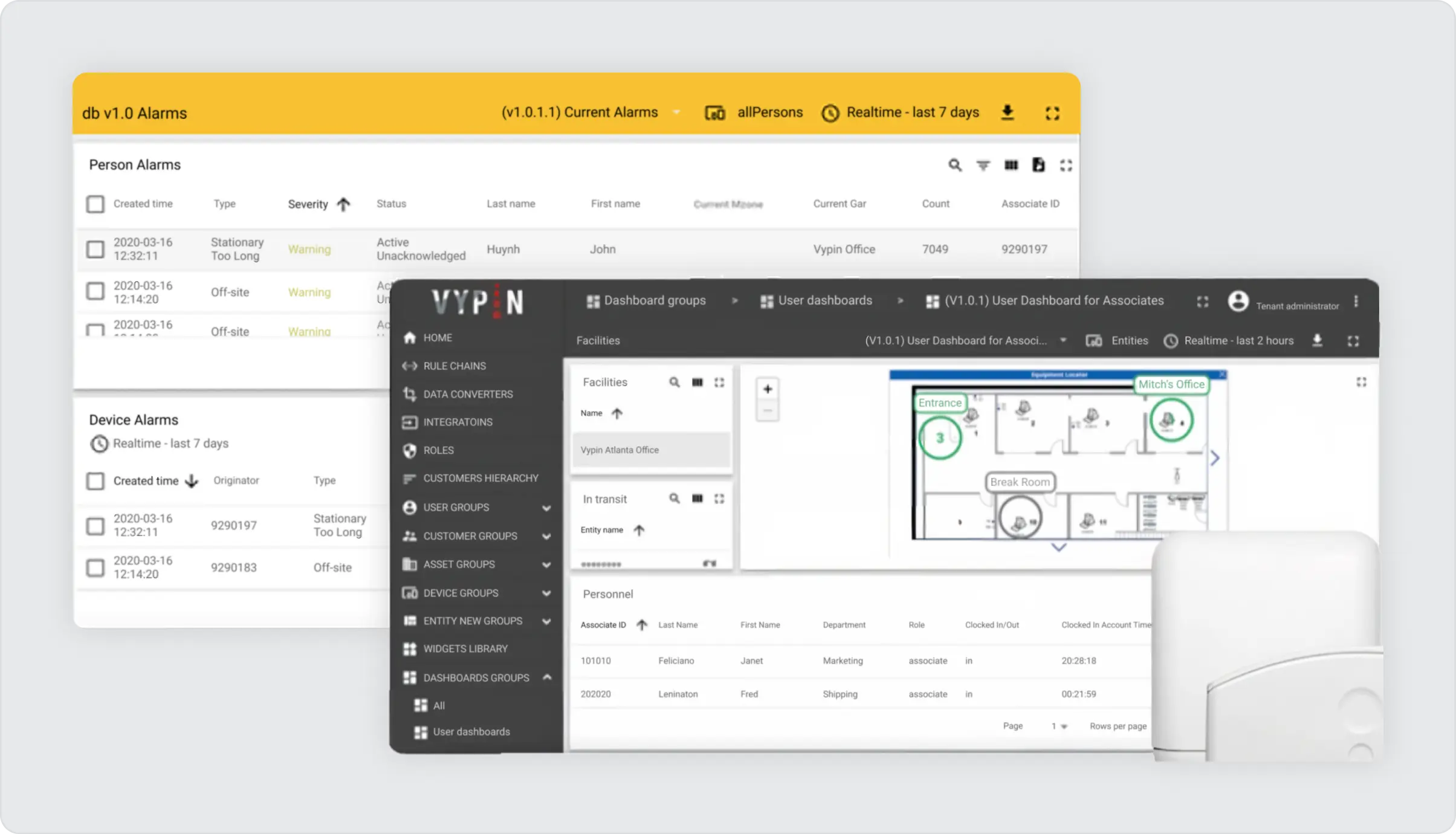
Accessing the dashboard, which displays various graphs and tables, simply requires logging into the system from a web browser.
A noteworthy feature is the customized notification system, established through ThingsBoard’s rule engine and device profiles. This system alerts the relevant individuals in case of deviations from preset conditions, such as temperature threshold breaches. This feature facilitates immediate response and the implementation of preventive measures.
The outcome
Prevention is universally acknowledged as more efficient, cost-effective, and less time-consuming than cure. With the newly installed warehouse monitoring system, the client now has the capability to proactively prevent issues before they arise, ensuring the safety and integrity of their sensitive products.
This case study exemplifies the power of IoT in enhancing regulatory compliance and operational efficiency in critical industries such as pharmaceuticals. The Vypin-WhereView solution, backed by ThingsBoard, demonstrates our commitment to delivering innovative and reliable monitoring solutions.